Biosimilars have already proven that they can generate considerable cost savings if they’re managed and used appropriately, and some patients are already benefiting from increased access as a result of these products.1 We are on a mission to make biosimilars “mainstream,” meaning anyone can access them in the U.S. market. And we are uniquely positioned to accomplish this goal.
Backed by the resources of our parent company, Intas Pharmaceuticals, we’re able to maintain an entrepreneurial mindset with the resources and manufacturing capabilities to contribute towards better healthcare through innovation.

Pharmaceutical company with rapid decision-making and a focus on long-term returns
The right approach, the right improvements, the right medicines delivered in the right way at the right time. That’s our culture. And it has been since the formation of our parent company, Intas Pharmaceuticals, in 1976. As one of the pioneers and market leaders of biosimilars, having launched its first product in India in 2004, Intas has long been ahead of the game in this space.
Developing, manufacturing, and marketing biologics for more than 20 years with one of India’s largest r-protein fermentation capacities
Since their inception, Intas has grown to employ 19,000+ team members and has spread to reach patients in over 85 countries, with 60% of that impact coming from the EU and U.S. And they are consistently one of the most recognized companies in industry awards.

Pharmaceutical company with rapid decision making and a focus on long-term returns
Developing, manufacturing, and marketing biologics for more than 20 years with one of India’s largest r-protein fermentation capacities
19,000+ employees including 7,500+ commercial staff globally
On this platform, Intas launched its Global Biologics Business Unit in 2000 with a focus on developing high-quality recombinant DNA protein products and plasma-derived products. That work has led to:
Sales derived from 85+ countries with ~60% from EU and U.S.
Intas is leveraging a transformative pipeline with diverse targets, using an aggressive development and go-to market strategy, and has a number of products both in development and in filing.
And Intas is aiming to become a top-10 global player investing in the specialty pharmaceuticals market.
Over $450M
investment in sites and
new initiatives
Over $450M
investment in sites and
new initiatives
We’ve been here before.
Accord BioPharma’s president, Chrys Kokino, has witnessed the growth of the biosimilar market since it began. He believes in the impact these drugs can create—and his vision for biosimilar adoption is mirrored throughout the organization.
“When I was working for a big pharma company around the time biosimilars began, my peers and I were all a little skeptical of what biosimilars would bring to the markets, especially since there were so many unknowns around how they were developed.”
“But when a regulatory approval pathway was created and education increased, biosimilars underwent a drastic evolution. And when I realized their promise and potential, I decided to get in on the ground floor. We at Accord BioPharma are investing heavily in the development of biosimilars to drive patient- and provider-centric solutions.”
We may be new to the game as a company, but our people have been here for some time. Many of our team members have helped to launch biosimilars in Europe, long before the healthcare industry had the ability to introduce biosimilars in the U.S., and they understand what it takes to bring these products to market. Leveraging a diverse senior leadership team with more than 125 years of combined experience across multiple therapeutic areas, Accord BioPharma has the experience and energy to activate on our insights and deliver for the stakeholders we serve.
Our culture is patients first. Always.
At all levels of the Accord BioPharma organization, we’re committed to the people we serve first and foremost. We have hand-selected all the senior leaders within our organization with a focus on their prior experience commercializing products in our therapeutic areas of focus.
We want fierce advocates for our portfolio with a wealth of experience and exposure. Our people understand the challenges certain medications will face, and they share a passion for introducing our products. They want to make a difference.
That work begins by analyzing the current marketplace. Accord BioPharma holistically examines treatment options to consider how we can take a product that’s currently out there and fine tune it to deliver it in the most cost-effective or consumer-friendly way. We want to ensure that the administration, tracking, and follow-up are just as reliable as the drug itself.
We do everything we possibly can to provide the right information and the right treatment options for the right patients. The premise of trying to drive business is not our primary concern—but rather what we can do to help patients or providers. And that’s reflected in the mindset of every person at our organization.
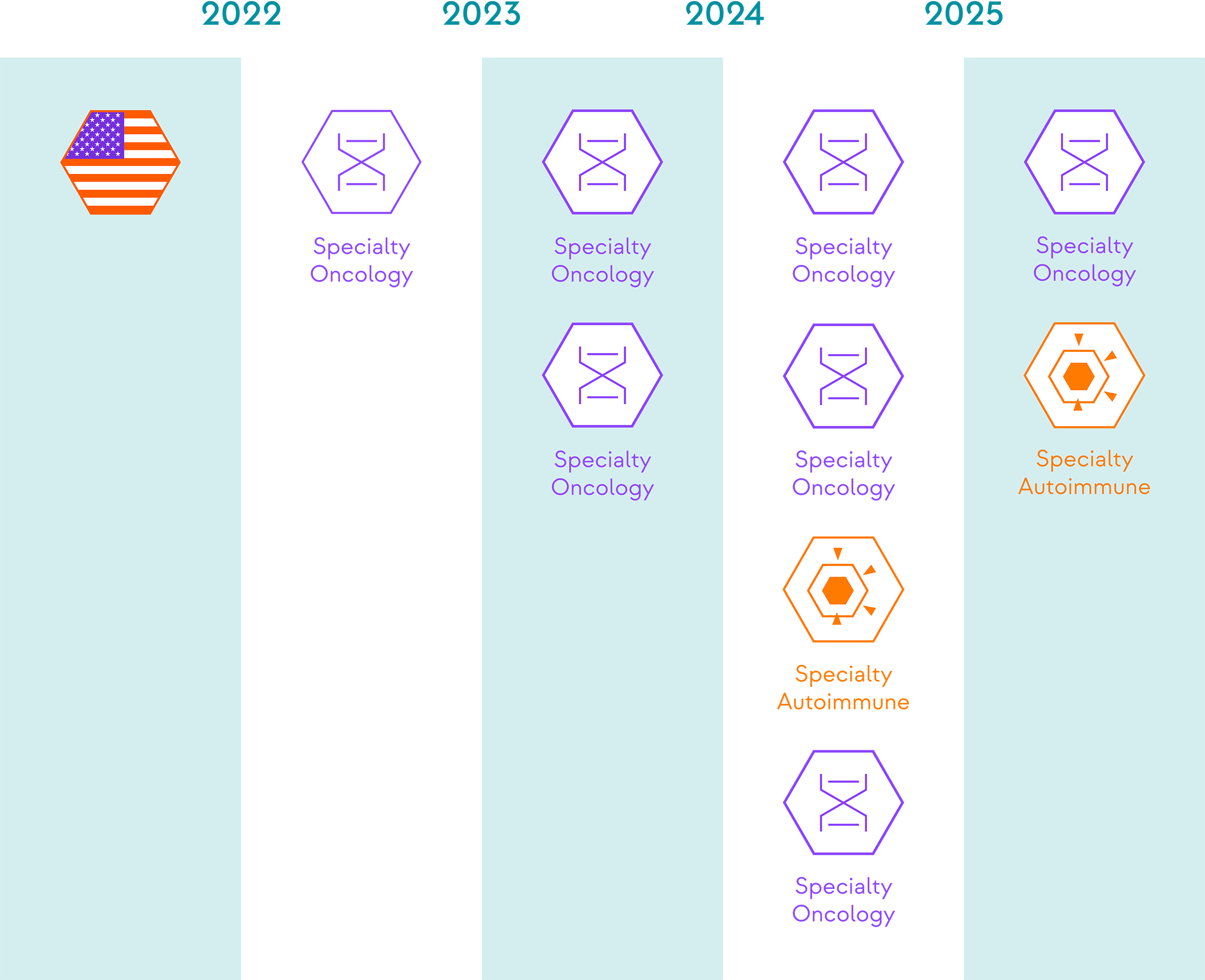
*Launch dates subject to change.

“My personal belief is that biosimilars are here to stay. They have already proven there are considerable cost savings and access benefits if they’re managed and used appropriately, and that without a doubt some patients are benefiting tremendously from these products.”
Chrys Kokino, Company President
Want to know more about biosimilars and how they’re made? Visit our Understanding Biosimilars page to get answers to some of the most common biosimilars questions.
For more on the impact biosimilars can create and Accord BioPharma’s story, visit the following pages:
To learn more about the promise of biosimilars, the impact they can create, and our work in this space, visit the following pages:
1. Biosimilars in the United States 2023-2027. IQVIA. https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-unitedstates-2023-2027. Published January 31, 2023.
2. Kavanagh C. Paragraph IV filings, Generic Drugs and Big Pharma. Minesoft. May 24, 2022. https://minesoft.com/paragraph-iv-filings-genericdrugs-big-pharma/.
Do not use CAMCEVI® in patients with hypersensitivity to GnRH, GnRH agonist analogs, or any of the components of CAMCEVI as anaphylactic reactions to these drugs have been reported in the medical literature.
CAMCEVI, like other GnRH agonists, causes a transient increase in serum levels of testosterone during the first week of treatment which can cause transient worsening of symptoms. As with other GnRH agonists, cases of ureteral obstruction and spinal cord compression, have been observed, which may contribute to paralysis with or without fatal complications.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy.
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Blood glucose levels should be monitored and managed according to current clinical practice.
Increased risk of myocardial infarction, sudden cardiac death, and stroke has been reported in association with the use of GnRH agonists. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients should be monitored for cardiovascular disease and be managed according to current clinical practice.
Androgen deprivation therapy may prolong the QT/QTc interval. Consider periodic monitoring of electrocardiograms and electrolytes.
Convulsions have been reported in patients receiving GnRH agonists, like CAMCEVI. Patients experiencing convulsions should be managed according to the current clinical practice.
Monitor serum levels of testosterone following injection of CAMCEVI.
Based on findings in animal studies and mechanism of action, CAMCEVI may cause fetal harm when administered to pregnant women.
The most common (≥10%) adverse reactions during a median follow-up of 336 days were hot flush, hypertension, injection site reactions, upper respiratory tract infections, musculoskeletal pain, fatigue, and pain in extremity.
CAMCEVI is indicated for the treatment of adult patients with advanced prostate cancer.
Click here for full Prescribing Information.
©2024 Accord BioPharma, Inc. All rights reserved WS-ABI-2200001
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other." |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |